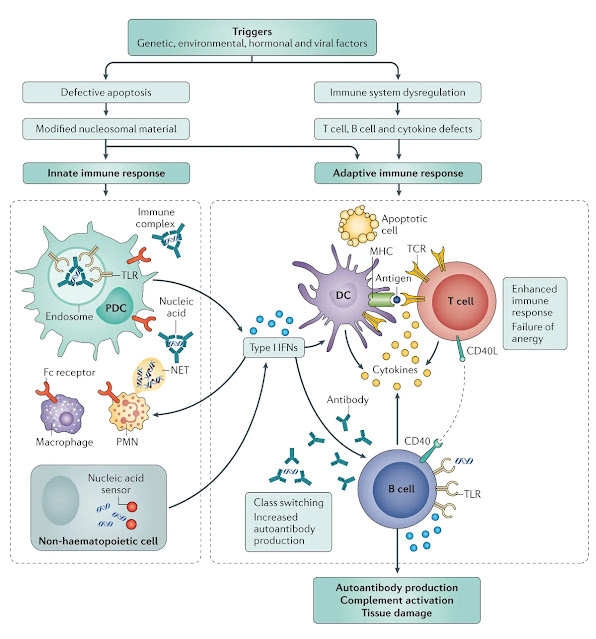
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterised by multisystem involvement and a complex interplay of genetic, environmental, and immunological factors. It primarily affects young women and can manifest with diverse clinical symptoms, ranging from mild cutaneous involvement to life-threatening organ damage. Understanding its pathophysiology is crucial for developing targeted therapies and improving patient outcomes.
Hypersensitivity classification
SLE is classified as a type III hypersensitivity reaction. This means that immune complexes—formed by autoantibodies binding to self-antigens—deposit in tissues, triggering complement activation and inflammation. This leads to widespread tissue damage, particularly in the kidneys, skin, joints, and blood vessels.
Additionally, some aspects of SLE involve type II hypersensitivity, where autoantibodies directly target cells, leading to haemolysis and cytotoxic effects. This is particularly relevant in haematological manifestations, such as autoimmune haemolytic anaemia and thrombocytopenia.
Immune dysregulation and loss of self-tolerance
- Genetic predisposition: Certain HLA haplotypes and polymorphisms in immune regulatory genes increase susceptibility to SLE.
- Environmental triggers: UV radiation, infections, and hormonal influences can initiate immune dysregulation.
- Epigenetic modifications: Aberrant DNA methylation and histone modifications alter gene expression, promoting autoimmunity.
Autoantibodies and diagnostic criteria in Australia
- Antinuclear antibodies (ANA) – Present in >95% of SLE cases, making it the screening test for lupus. However, ANA can be positive in other autoimmune diseases, meaning additional specific autoantibody testing is required for confirmation.
- Anti-double-stranded DNA (anti-dsDNA) – Highly specific for SLE and correlates with disease activity, particularly in lupus nephritis. Higher titres suggest renal involvement and increased inflammation.
- Anti-Smith (anti-Sm) antibodies – Specific for SLE but found in only ~30% of patients. These antibodies are associated with renal disease and severe systemic manifestations.
- Antiphospholipid antibodies – Includes lupus anticoagulant, anticardiolipin, and anti-beta2 glycoprotein I, contributing to thrombosis and pregnancy complications (e.g. recurrent miscarriage).
- Complement levels (C3, C4) – Low levels indicate active disease, as complement is consumed by immune complex deposition.
Additional autoantibodies in SLE
- Anti-Ro (SSA) and Anti-La (SSB) antibodies – Associated with cutaneous lupus, photosensitivity, and neonatal lupus (risk of congenital heart block).
- Anti-RNP antibodies – Found in mixed connective tissue disease (MCTD) but also present in SLE, contributing to Raynaud’s phenomenon and arthritis.
- Anti-histone antibodies – Strongly linked to drug-induced lupus, particularly from medications like hydralazine, procainamide, and isoniazid.
- Anti-nucleosome antibodies – Emerging as a sensitive marker for lupus nephritis, often correlating with renal disease activity.
- Anti-C1q antibodies – Implicated in severe lupus nephritis, particularly in patients with low complement levels.
- Rheumatoid factor (RF) – Occasionally present in SLE, contributing to overlapping features with rheumatoid arthritis.
Clinical relevance and diagnostic considerations
- Anti-dsDNA and low complement levels indicate active lupus nephritis.
- Anti-Ro/SSA antibodies warrant monitoring in pregnancy due to the risk of neonatal lupus.
- Antiphospholipid antibodies necessitate thrombosis prevention strategies, including anticoagulation therapy.
Organ-specific pathophysiology
- Renal involvement (lupus nephritis): Immune complex deposition in glomeruli leads to inflammation, proteinuria, and progressive renal dysfunction.
- Cutaneous manifestations: Photosensitivity and immune-mediated skin damage result in characteristic rashes, including the butterfly rash.
- Neurological complications: Autoantibody-mediated neuronal damage contributes to cognitive dysfunction, seizures, and psychiatric symptoms.
- Cardiovascular effects: Chronic inflammation accelerates atherosclerosis, increasing the risk of cardiovascular disease.
Clinical cases
Case 1: Young woman with lupus nephritis
Case 2: Medical student with fatigue and arthralgia
Case 3: Middle-aged woman with APS and stroke
Biologic therapies for systemic lupus erythematosus (SLE)
Belimumab – A monoclonal antibody targeting B-cell activating factor (BAFF), reducing B-cell survival and autoantibody production.
- Mechanism: Inhibits excessive B-cell activation, decreasing autoantibody-mediated tissue damage.
- Indications: Approved for active, autoantibody-positive SLE in Australia, particularly for patients with persistent disease despite standard therapy.
Anifrolumab – A monoclonal antibody targeting type I interferon receptor, blocking interferon-mediated immune activation.
- Mechanism: Suppresses interferon-driven inflammation, reducing disease flares and organ damage.
- Indications: Recently listed on the Pharmaceutical Benefits Scheme (PBS) in Australia for moderate to severe SLE, providing affordable access for eligible patients.
Rituximab – A monoclonal antibody targeting CD20 on B cells, leading to B-cell depletion.
- Mechanism: Reduces autoantibody-producing B cells, dampening immune-mediated tissue damage.
- Indications: Used in refractory lupus nephritis and severe SLE manifestations, particularly in patients who fail conventional therapy.
Clinical considerations in Australia
- Patient selection: Biologics are typically reserved for patients with high disease activity, particularly those with lupus nephritis, refractory arthritis, or severe cutaneous involvement.
- Combination therapy: Biologics are often used alongside hydroxychloroquine and corticosteroids, allowing for steroid-sparing effects and improved long-term disease control.
- Safety considerations: Patients receiving biologics require regular monitoring for infections, infusion reactions, and potential adverse effects.




No comments:
Post a Comment